CBSE Class 10 Science Chapter 4 Carbon and its Compound Important Question Answers of 1,2,3 and 5 Markers
Carbon and its Compound Important Questions – Here are the important questions (1,2,3 and 5 Marks) for CBSE Class 10 Science Chapter 4 Carbon and Its Compounds. The important questions we have compiled will help the students to brush up on their knowledge about the subject. Students can practice Class 10 Science important questions to understand the subject better and improve their performance in the board exam. The solutions provided here will also give students an idea about how to write the answers. Take Free Online MCQ Test for Class 10
Video of Carbon and its Compound Important Questions
| Carbon and its Compound MCQs |
| 1 Mark Questions |
| 2 Mark Questions |
| 3 Mark Questions |
| 5 Mark Questions |
Class 10 Science Carbon and its Compound MCQs (1 Marks)
1. In diamond, each carbon atom is bonded to four other carbon atoms to form
a) a hexagonal array
b) a rigid three-dimensional structure
c) a structure in the shape of a football
d) a structure of a ring
Answer – b
2. Name the functional group present in CH3COCH3.
a) Alcohol
b) Carboxylic acid
c) Ketone
d) Aldehyde
Answer – c
3. Why does carbon form compounds mainly by covalent bonding?
a) There are four electrons in the outermost shell of carbon.
b) It requires a large amount of energy to form C4+ or C4sup>4-.
c) It shares its valence electrons to complete its octet.
d) All the above.
Answer – d
4. A hydrocarbon has four carbon atoms. Give its molecular formula if it is an alkene.
a) C4H10
b) C4H8
c) C4H8
d) C4H4
Answer – b
Carbon and its Compound Important Questions Video
Carbon and its Compound Important Question of 1 Marks Very Short Answer Questions
Q. 1- How many covalent bonds are there in a molecule of ethane, C2H6?
Answer – There are seven covalent bonds in a molecule of ethane:
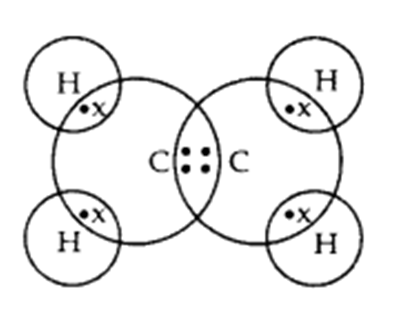
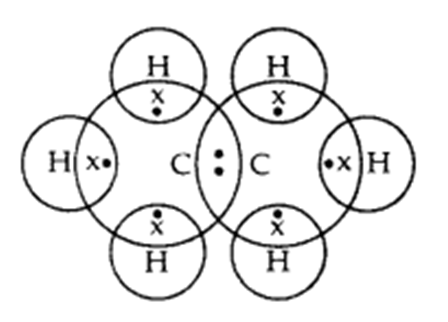
Q. 2 – Write the electron dot structure of ethane molecule (C2H4)
Answer – Electron dot structure of ethane molecule, C2H4:
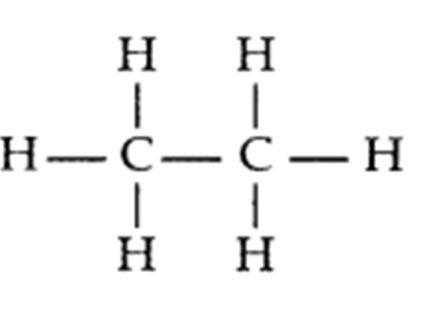
Q. 3 – Write the electron dot structure of ethane molecule, C2H6.
Answer – Electron dot structure of ethane, C2H6:
Q. 4 – Draw the structure of a hexanal molecule, C5H11CHO.
Ans. Hexanal molecule, C5H11CHO:
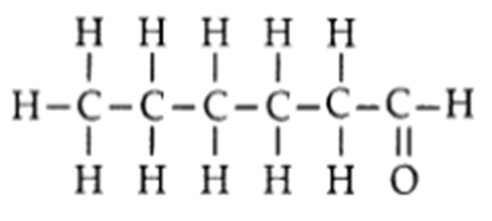
Q. Name the functional group present in each of the following compounds:
a. CH3COCH3
b. C2H5COOH
Answer
| Organic Compound | Functional Group |
| (i) CH3COCH3 | (>C = O) Ketonic Group |
| (ii) C2H5COOH | (– COOH) Carboxylic Acid |
Carbon and its Compound Related Links –
| Carbon and its Compound Lesson Explanation | Carbon and its Compound MCQs |
| Carbon and its Compound Video Explanation | Carbon and its Compound MCQs Video |
Carbon and its Compound Important Question of 2 Marks Short Answer Type Questions
Q. 1 – Name the main products formed when
(i) Ethanol is oxidised by an alkaline solution of KMnO4
(ii) Ethanol reacts with ethanoic acid.
(iii) Sodium ethanoate is heated with soda lime.
(iv) Ethanol is heated with cone. H2SO4 acid.
Ans – (i) Ethanoic acid
(ii) Ethyl ethanoate
(iii) Methane (CH4) gas
(iv) Ethene gas
Carbon and its Compound Important Question of 3 Marks Short Answer Type Questions
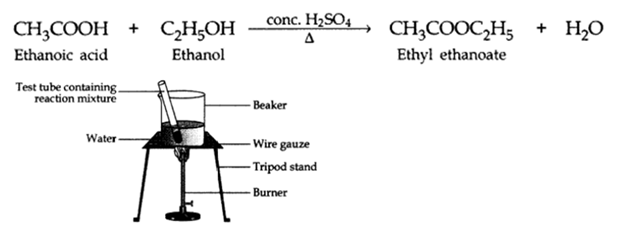
Q2. What is an ‘esterification’ reaction? Describe an activity to show esterification.
Ans – Carboxylic acid when reacts with alcohols in the presence of concentrated sulphuric acid to form pleasant smelling esters.
This reaction is called an esterification reaction.
The formation of esters from acid and an alcohol can be demonstrated by the following experiment:
Activity:
- About 2 – 3 ml of ethanol is taken in a test tube.
- About 2 – 3 ml of glacial acetic acid along with a few drops of concentrated sulphuric acid is added to the test tube.
- The reaction mixture is then heated in a water bath for two minutes.
- Now the contents are poured into a beaker containing 20 – 50 ml water. Notice the smell.
- We will observe sweet smell due to the formation of
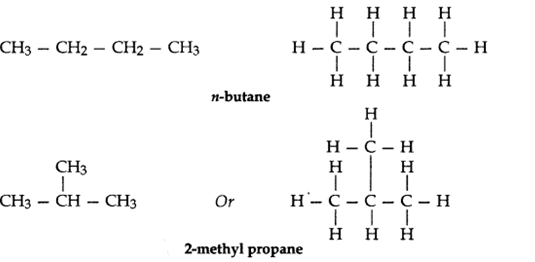
Q3. – What are isomers? Draw the structures of two isomers of butane, C4H10.
Why can’t we have isomers of first three members of alkane series?
Ans – The organic compounds having the same molecular formula but different structures are known as isomers. Isomers of butane, C4H10 :
Isomerism is not possible in first three members of alkane series (i.e., methane, ethane, propane) because they contain only one, two or three carbon atoms respectively and with only 1, 2 or 3 carbon atoms, it is not possible to have different arrangements of carbon atoms.
Important Videos Links
Carbon and its Compound Important Question of 5 Marks Very Long Answer Type Questions
Q1. (A) Differentiate between alkanes and alkenes. Name and draw the structure of one member of each.
(B) Alkanes generally burn with clean flame. Why?
Ans –
Alkanes:
- An alkane is a hydrocarbon in which the carbon atoms are connected by only a single covalent bond.
- General formula of alkane is CnH2n+2.
- The simplest alkane is methane (CH4).
- Alkanes generally bum in air with a blue and non -sooty flame.
- Alkanes undergo substitution reactions.
- Alkanes do not decolourise red-brown colour of bromine water.
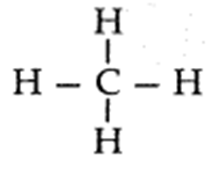
- An alkene is an unsaturated hydro-carbon in which the two carbon atoms are connected by a double bond.
- General formula of alkene is CnH2n.
- The simplest alkene is ethene (C2H4).
- Alkenes burn in air with a yellow and sooty flame.
- Alkenes undergo addition reactions.
- Alkenes decolourise the bromine water.
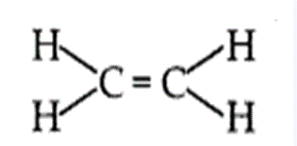
(b) Alkanes burn in air with a blue and non-sooty flame because the percentage of carbon in the alkane is comparatively low which gets oxidised completely by oxygen present in air.
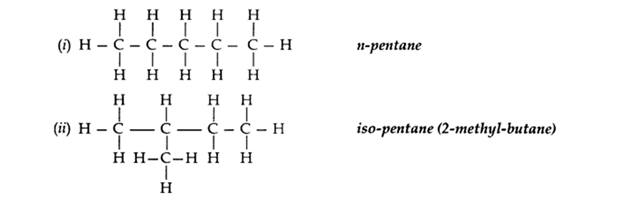
Q2. What is meant by isomers? “We cannot have isomers of the first three members of the alkane series.”
Give reason to justify this statement. Draw the structures of two isomers of pentane, C5H12.
Ans – The organic compounds having the same molecular formula but different structures are known as isomers.
Isomers of the first three members of alkane series are not possible because only one arrangement of carbon atoms is possible in their molecules.
Two isomers of pentane C5H12:
Q3. What are esters? How are they prepared? List two uses of esters.
Ans – Esters are usually volatile liquids having pleasant smell (in fruity smell).
Preparation: When carboxylic acid reacts with an alcohol in the presence of a little concentrated sulphuric acid, it gives a pleasant smelling ester.
For Example: When ethanoic acid is warmed with ethanol in the presence of a few drops of concentrated sulphuric acid, a sweet smelling ester called ethyl ethanoate is formed.
Uses:
Esters are used in making perfumes.
Esters are used in making artificial flavours and essences used in icecreams, sweets and cold drinks.
Q4. What are homologous series of carbon compounds? Write the molecular formula of two consecutive members of homologous series of aldehydes. State which part of these compounds determines their:
(i) Physical and
(ii) Chemical Properties.
Homologous Series: A series of carbon compounds in which the same functional group substitutes for hydrogen on a carbon chain is called a homologous series.
There is a difference of – CH2 in the molecular formulae of two nearest compounds of a homologous series.
Each such series has the same general molecular formula and has a general scientific name. There is a difference

(a) In a tabular form, difference between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
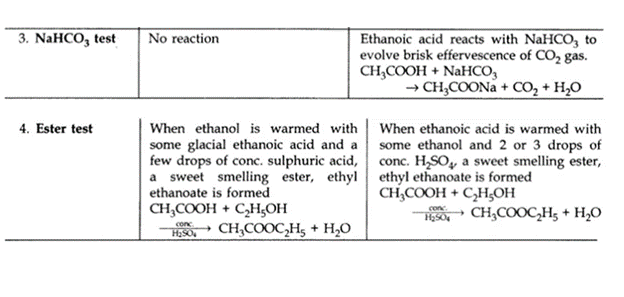
(iii) NaHCO3 test
(iv) Ester test
Ans –
(b) Write a chemical reaction to show the dehydration of ethanol.
| Ethanol | Ethanoic acid | |
| 1. Physical state | Liquid state | Liquid state |
| 2. Taste | Burning bitter | Sour |